Sprayable Postoperative Adhesion Barrier
Postoperative adhesions are “expected outcomes” in abdominal surgeries, forming after 90% of surgical procedures causing chronic debilitating pain and increasing the complexity of future surgeries.
Critically, 1 in 3 births in the United States happens by cesarean section. These surgical procedures have a 5x increase in the formation of postoperative adhesions, which are a leading cause of infertility in women and complicate future pregnancies and deliveries.

In response, Luna Labs has developed a sprayable hydrogel barrier, AeroVeil™, that will provide surgeons with an effective, inexpensive, and safe tool to reduce the incidence and severity of postoperative adhesions.
AeroVeil conforms to tissue, is colored for easy visualization, biodegrades, and maintains a barrier for 4-7 days until the risk of adhesions has passed.
Pre-clinical efficacy testing in rats and rabbits demonstrated reduction in the incidence and severity of postoperative adhesion formation. Product biocompatibility was demonstrated under ISO10993 to support product transition and regulatory review.
 Untreated Control
Untreated Control
 AeroVeil Treatment
AeroVeil Treatment
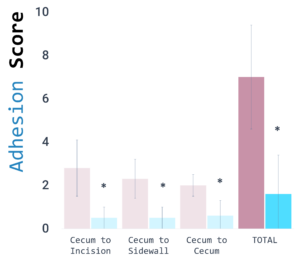
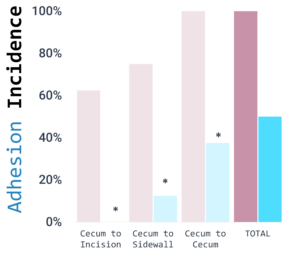
Reduced adhesion incidence and severity score in a rabbit model of adhesion formation





This product is protected under European Patent EP3606572 and is under review in the US and China under PCT/US2018/024716.
Get the latest news from Luna Labs' biotech, materials, systems, and medical simulation teams.